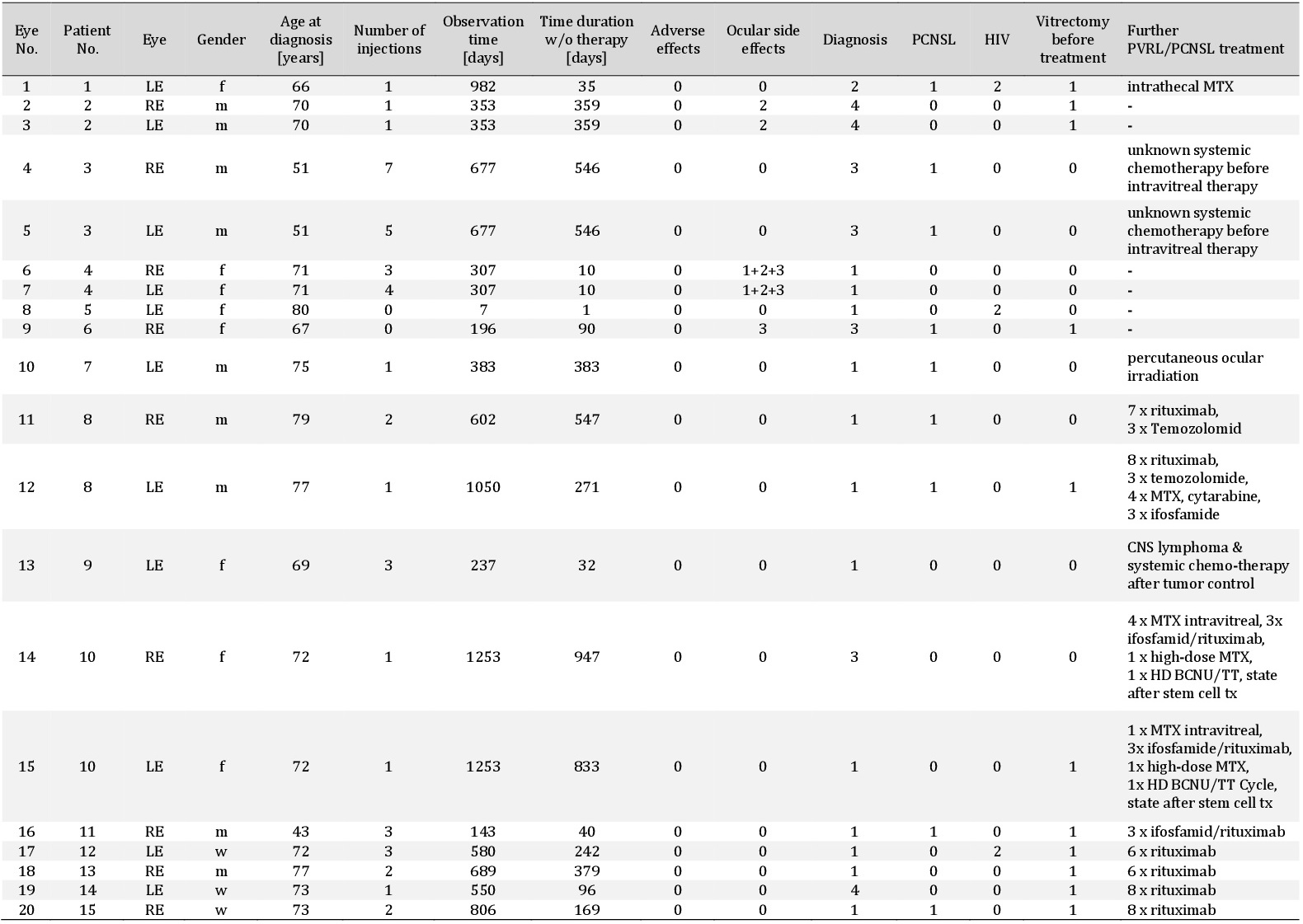
Table 1. Biodata: PVRL patients' parameter. Biodata as well as clinical parameters for intravitreal rituximab treatment are noted for all 20 eyes from 15 PVRL patients. Eye (RE=right eye; LE=left eye), gender (m=male), adverse effects (0=not described), ocular side effects (0=not described, 1=elevated intraocular pressure; 2=cataract; 3=retinal detachment), diagnosis (0=unknown, 1=vitreous biopsy, 2=CNS, 3=clinical diagnosis; 4=retinal biopsy), PCNSL (0=no CNS lymphoma, 1=CNS lymphoma), HIV (0=no, 1=yes), vitrectomy before treatment (0=no vitrectomy before treatment, 1= vitrectomy before treatment), MTX=methotrexate, BCNN/TT=carnemustine/thiotepa